 Sabtu, 8 Februari 2025 (18:04)
Sabtu, 8 Februari 2025 (18:04)
 Music |
 Video |
 Movies |
 Chart |
 Show |
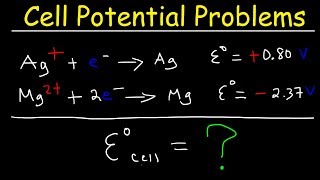 |
Cell Potential Problems - Electrochemistry (The Organic Chemistry Tutor) View |
 |
For a standard cell Zn(s)|Zn^(2+)(1 M)||Cu^(2+)(1 M)|Cu(s) Write the electrode reaction and cell... (Doubtnut) View |
 |
Cell Voltages Non-Standard Conditions (MooreChemistry) View |
 |
Electrochemistry (Professor Dave Explains) View |
 |
Voltaic cell | How does it work (Sabins) View |
 |
How to Calculate Standard Cell Potential and Voltage using E cell = E cathode - E anode Examples (Conquer Chemistry) View |
 |
The `e.m.f.` of a cell corresponding to the reaction : `Zn ((s))+2H ((aq.))^(+) rarr underset((0.1 (Doubtnut) View |
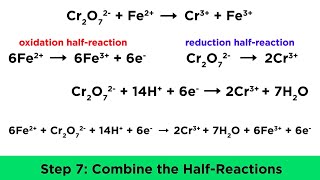 |
Balancing Redox Reactions in Acidic and Basic Conditions (Professor Dave Explains) View |
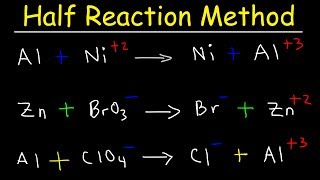 |
Half Reaction Method, Balancing Redox Reactions In Basic u0026 Acidic Solution, Chemistry (The Organic Chemistry Tutor) View |
 |
Two electrochemical cell , Zn|Zn2+ ||Cu2+ |Cu and Fe|Fe2+ ||Cu2+ |Cu are connected in series. emf (PB Edu) View |